Into the Laboratory, it’s time to go to work.
Next, let’s take a look CO2 from an Atmospheric Physicist‘s view – straightforward physics that we hope most of you will be able to follow:
What we commonly call “light” is actually electromagnetic radiation, physically no different from radio waves, except of different frequencies and wavelengths. The part we can see is called the visible spectrum. Beyond what we can see in the higher frequencies ( and shorter wavelengths, since they are reciprocal functions ) lies the ultraviolet spectrum. UV light is very penetrating, which is why one could get sunburned on an overcast day. Beyond even that are X-rays, which can penetrate much deeper. On the opposite end of the visible spectrum lies infra-red… which you can’t see, but you can easily feel, as anyone who has warmed his hands near a hot stove can testify. It is the infrared portion we commonly refer to as “heat” radiation. And beyond that are the radio and television wavelengths we all know and love.
The sun is very “bright”, and its frequency spectrum is generally too short to produce much infrared coming down through the atmosphere. Radiation from the sun penetrates the atmosphere, strikes the earth, and some of it is absorbed and some is reflected. The different bandwidths (colors) of reflected light depend on the material struck, so something green-colored is reflecting the green portion of the visible spectrum and absorbing the rest. This heats up the earth, and that’s the first part of the story.
All heated bodies emit radiation in the infrared range. This is called “black body” radiation, because a perfectly black body reflects no visible light but still emits radiation in a specified band of wavelengths. Infrared radiation is of a much longer wavelength, and can be much easier absorbed by certain components in the atmosphere, causing them to also “heat up”. The warm air around us is being kept warm partially from black body radiation coming from the earth itself. Another method of warming is by conduction – air coming in contact with the heated soil, rocks, trees, buildings, etc. and being directly warmed by that contact. This may be a bigger factor than we think, but we’re not going to attempt to try to determine just how much that might be. We’d have to know the total surface area of every object – down to the smallest blade of grass – there is on our planet. We also need to remind ourselves that there is actually no physical quantity known as “cold”. There is only “heat” and “lack of heat”.
Next, lets talk about a scientific process called Atomic Absorption Spectrometry. It is a method by which we can measure precisely which wavelengths of radiation a particular gas is capable of absorbing.
In our highly simplified drawing above, a radiation source is beamed through a glass container containing a gas sample. As the radiation passes through, a portion of it is absorbed at particular narrow bandwidths (often more than one ) so the end result are some “missing” sections of the whole spectrum coming from the source, which show up as dark lines. They’re missing because they were absorbed by the sample in the chamber. They are called absorption lines, or absorption spectra, and when analyzed by a knowledgeable person, can tell one what the gas or gas mixture is in the sample chamber based on a catalog of known spectra. It’s a wonderful tool for analyzing unknown gas samples.
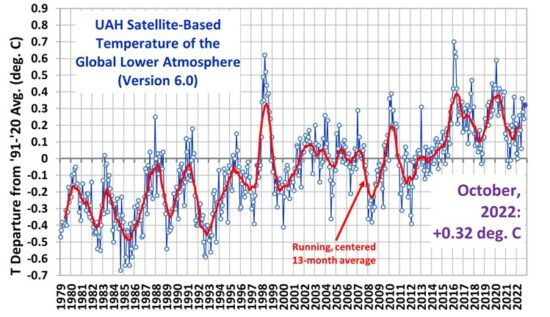
Let’s look at a real result, below – the absorption spectrum for pure carbon dioxide plus an amount of water vapor equal to that in our current atmosphere as the sample and infrared radiation from a black body spectrum as the source. This is part of the so-called “greenhouse effect”
As we can see above, carbon dioxide absorbs infrared radiation (IR) in only three narrow bands of frequencies, which correspond to wavelengths of 2.7, 4.3 and 15 micrometers (µm), respectively. The percentage absorption of all three lines combined can be very generously estimated at about 8% of the whole IR spectrum, which means that 92% of the “heat” passes right through without being absorbed by CO2. In reality, the two smaller peaks don’t account for much, since they lie in an energy range that is much smaller than the where the 15 micron peak sits – so 4% or 5% might be closer to reality. If the entire atmosphere were composed of nothing but CO2, i.e., was pure CO2 and nothing else, it would still only be able to absorb no more than 8% of the heat radiating from the earth.
| Note: In our original draft, we talked a bit about relative spacing geometry, to give the reader a feel for the distance between molecules in the atmosphere. We talked in (very crude) terms about tacking bottle caps up on a barn wall, and gave some spacing examples in 2 dimensions for a rough feel of the subject. One of our readers, Peter J. Morgan – a consulting engineer from New Zealand – undertook to re-write our simple ( and not technically accurate ) description for his 15 year old son. He was kind enough to send it to us, and we liked it so much we threw out our South Park estimate and substituted his work instead. Thanks, Peter! |
To give you a feeling for how little CO2 there actually is in the atmosphere, let’s note that atoms and molecules are very tiny things, and the distances between them are therefore also very small. Physicists like to use a unit of measure called an Angstrom, which is 0.1 of a nano-meter, or a 0.1 billionth of a meter, (i.e. 10-10 of a meter or 10-7 of a mm). A molecule like CO2 has a size of around two Angstroms (2 x 10-7 mm). The density of the gas is 10 to the 24th power number of molecules occupying a space of about 22 liters (i.e. 4.55 x 1022 molecules per liter) at a pressure of 760mm of mercury and 273 degrees Kelvin (i.e. 32 degrees Fahrenheit or zero degrees Celsius) – called the “standard temperature and pressure”. You can almost think of all this as just the normal temperature and pressure around you right now. A simple calculation shows that in a 3-dimensional tetrahedron array, as shown in the diagram below (for the closest possible packing with an equal distance between molecules), the spacing between molecules is approximately 28 Angstroms.
For equidistant packing, a tetrahedron arrangement is required
To fit 4.55 x 1022 molecules equispaced in a 100-mm cube (i.e. one liter) they have to be 28 Angstroms apart.
Since at 2 x 10-7 mm diameter, CO2 is a very tiny molecule, let’s magnify the picture by a factor of 10 million, so that we can imagine a CO2 molecule as a 20 mm diameter marble floating in the air. However, CO2 makes up only 380 of each million molecules of air – the rest are a mixture of all the other atmospheric gases and water vapor – i.e. only one in every 2632 molecules is a CO2 molecule. Let’s imagine that all the other molecules are colored blue, and CO2 molecules are colored red. All the marbles making up our model atmosphere are equispaced at 280 mm apart. When mixed evenly into our model atmosphere (which is what the wind does) a bit more simple math shows that our red marbles are equispaced at 3900 mm (i.e. 3.9 meters) apart. In the real atmosphere, at a height of approx. 5500 meters, pressure is halved from what it is at sea level. A bit more simple math shows that at a height of 5500 meters (55 million kilometers in our model – that’s 143 times the distance from earth to the moon!), our 20 mm diameter CO2 marbles are equispaced at 4.9 meters apart. Now you know why CO2 is called a “trace” gas.
This whole picture we have drawn ( with Peter Morgan’s help ) illustrates both how little CO2 there is in the atmosphere, and how relatively little of the radiation it is capable of absorbing and “heating” the atmosphere. We know that most of the other IR radiation bands slips through and doesn’t get to do any heating at all. (We’ve all seen the nice IR photographs taken from the space station.) But some scientists such as Dr. Heinz Hug who specialize in study of this stuff claims that all of the heat in these particular spectra are indeed absorbed in a relatively short distance, so adding more CO2 to the atmosphere can’t affect anything at any rate. Other scientists, such as Dr. Roy W. Spencer at NASA – and one of the leading experts in the field of climate science – doesn’t completely agree
We’ve decided to be exceptionally generous to all concerned in the debate and look at the worst-case scenario, where we’ll say that all of the available heat in the CO2 absorption spectrum is actually captured. We know that man is responsible for about 3 % of it, so with the simplest of math, we have .03 x .08 = .0024. And remember that 8% figure was actually larger than reality, since the two side peaks don’t have much energy to capture.
Man-made CO2 doesn’t appear physically capable of absorbing much more than
two-thousandths of the radiated heat (IR) passing upward through the atmosphere.
And, if all of the available heat in that spectrum is indeed being captured by the current CO2 levels before leaving the atmosphere, then adding more CO2 to the atmosphere won’t matter a bit.
In short, the laws of physics don’t seem to allow CO2 it’s currently assumed place as a significant “greenhouse gas” based on present concentrations. The other “greenhouse gases” such as methane, nitrous oxide, tetrafluoromethane, hexafluoroethane, sulfur hexafluoride, trifluoromethane, 1,1,1,2-tetrafluoroethane, and 1,1-difluoroethane exist only in extraordinarily smaller amounts and aren’t even up for serious discussion by any segment of the scientific community. And, since the other components of the atmosphere (oxygen, nitrogen, and water vapor) aren’t materially affected by human activity, the “greenhouse effect” is essentially a totally natural phenomenon, unaffected by human activity. We could repeat the spectral analysis and calculations for Oxygen, or O2 ( The percentage of oxygen in the atmosphere remains exactly the same at all heights up to about 85 km, and is about 20.9% by volume ) and Nitrogen (N2) which is the whopper at 78.1% – but we won’t. We’ll leave that as your homework problem now that you know how to do it. Just look up the atomic absorption spectra for both, and do the math. You’ll discover that Oxygen and Nitrogen aren’t even “greenhouse gases”, so that leaves the principal greenhouse gas… you guessed it…. Water Vapor. Curiously enough, the UN IPCC reports don’t even mention water vapor, since it is technically not a “gas” in the atmosphere. Dr. Roy W. Spencer has one of the best comments we’ve read on this subject:
| “Al Gore likes to say that mankind puts 70 million tons of carbon dioxide into the atmosphere every day. What he probably doesn’t know is that mother nature puts 24,000 times that amount of our main greenhouse gas — water vapor — into the atmosphere every day, and removes about the same amount every day. While this does not ‘prove’ that global warming is not manmade, it shows that weather systems have by far the greatest control over the Earth’s greenhouse effect, which is dominated by water vapor and clouds.” |
We can safely ballpark water vapor as being responsible for more than 95% of all the greenhouse effect, with oxygen and nitrogen playing no role and carbon dioxide being relatively insignificant… particularly the even smaller human-produced part.
Side note: Both Oxygen and Nitrogen don’t like to live alone. They prefer to find another and stick together into a diatomic ( 2 atom ) molecule. Thus the molecular weight of atmospheric oxygen or nitrogen is approximately twice that of one of them alone. We say “approximately”, because it takes energy to bind them together, and mass and energy are equivalent stuff, as our good friend Dr. Einstein explained with his famous equation E=MC2.
Now, you can sit back and give yourself a pat on the back, because you now know more pure physics of the atmosphere than a lot of so-called “climate scientists”, and likely know more than almost all of the non-scientist Popular Journalists and other writers churning out panic-stricken books and newspaper articles on the subject.
And for sure, you now know a lot more than Al Gore.
One would think this would be the end of the discussion, that the laws of physics show us that CO2 isn’t even a significant “greenhouse gas” and certainly the human contribution is insignificant. We both now know that CO2 can’t possibly be the evil byproduct all the ballyhoo has been claiming, and in fact, our biologist friends tell us if we could increase the CO2 content a little more, the planet would be much the richer… because plants love it, grow much larger with more of it, and we all like to eat. CO2 is a non-toxic, non-polluting, earth-friendly component that really is critical to our survival. Maybe that’s why we laughed so hard when the Popular Journalist in the Addison Independent insisted that 340, rather than 380 parts per million CO2 was a “target” we should all shoot for. While you’re pulling rabbits out of a hat, could you please bring me a Pepsi?
OK, if you still are compelled to worry about something, think about this: The amount of oxygen in our atmosphere is slowly diminishing. A very long time ago, it was as much as 35% of the atmosphere, and has been shrinking ever since. We always wondered why those plant-eating eating dinosaurs had such long necks, and now we know – they had to reach up for dinner into the really tall trees that once dotted our oxygen-rich planet.
But let’s not worry about that just now, for this current story is far from over. If you’ve read this far, you’re likely more curious than most, and probably more intelligent than average. And you probably want to know exactly what is causing the warming and cooling periods on the planet which have been going on for millennia. Inquiring minds want to know this stuff.
Continued at ‘Primer 4: ‘Bit of Background Aside’



Pingback: AGW – One man’s science is another man’s pseudo-science! Part 2. | The GOLDEN RULE
While you are correct that there is more eegrny in solar and wind. The problem is concentrating it enough for it to be useful and storing it in sufficient amounts to overcome the variability inherent in these modes. It is not a question of more research, it is a matter of physics.If you consider the path of development in almost all technologies the law of diminishing returns applies. That is to say the big advances are made at the beginning and subsequent ones return less and less improvement. In the case of wind and solar (and more importantly storage) this is what has happened. The gap between what we can do now, and what we would need to power an advanced civilization is simply too great to overcome and given that we know just about all there is to know about the theories that underpin these things, it is clear that it is unlikely that there will be a discovery that will allow that gap to be bridged.Now that’s not to say it’s impossible, just very unlikely and we need a source of clean power now. Waiting and hoping for a breakthrough that may not come in the situation that we are in now is just not possible. However no one is giving up on research and nuclear or not there will be people looking into these technologies, especially storage one way or the other, and if some discovery is made, and it leads to a cost effective technology, it will replace nuclear. But keep in mind that we are talking about a major discovery at the fundamental level, and these very rarely come from lavishing money on research.Your remark about weapons has been true: certainly wind, in the form of sailing ships, benefited from development for military reasons, but note to that the most efficient wind driven ships were those developed to carry tea – a commercial application. Reply
Thanks for this comment.
The main thing is to keep an open mind and have the welfare of the public at heart.
The underlying system failures and slowness in developing improvements are largely due to corporate greed.
More recent times this has been excacerbated by the world domination agenda.
From a civilization point of view the whole energy, financial, political, fast encompassing food, water and medical scenarios, are an unholy mess. Likely we are on the verge of a catastrophic civilization collapse.
Wow, wonderful blog structure! How lengthy have you beewn running a blog for?
you made blogging glance easy. The full look of your web site is fantastic, let alpone the content material!